1. Lithium batteries and nickel-cadmium and nickel-hydrogen rechargeable batteries:
The negative electrode of a lithium-ion battery consists of a graphite crystal, while the positive electrode is typically made of lithium dioxide. During the charging process, lithium ions move from the positive electrode to the negative electrode and are embedded in the graphite layers. When discharging occurs, lithium ions detach from the graphite crystal's surface and move towards the positive electrode. Consequently, during both charging and discharging, lithium exists solely in the form of lithium ions, never as metallic lithium. This type of battery is referred to as a lithium-ion battery or simply a lithium battery.
Lithium batteries boast several advantages, including small size, large capacity, lightweight, no environmental pollution, high single-cell voltage, low self-discharge rate, and numerous charge cycles. However, they are relatively expensive. Nickel-cadmium batteries are gradually being phased out due to their low capacity, significant self-discharge, and environmental pollution. NiMH batteries offer a high performance-to-price ratio and are environmentally friendly, but their cell voltage is only 1.2V, limiting their applications.
Second, the characteristics of lithium batteries:
1. They possess a higher energy density relative to their weight and volume;
2. The voltage is high, with each lithium-ion battery cell having a voltage of 3.6V, equivalent to the series voltage of three nickel-cadmium or nickel-hydrogen rechargeable batteries;
3. They have minimal self-discharge and can be stored for extended periods, which is one of their most outstanding features;
4. They lack a memory effect. Unlike nickel-cadmium batteries, lithium batteries do not require prior discharge before charging;
5. They have a long lifespan. Under normal operating conditions, lithium batteries can undergo more than 500 charge/discharge cycles;
6. They can be rapidly charged. Lithium batteries can typically be charged with a current of 0.5 to 1 times their capacity, reducing charging time to just 1 to 2 hours;
7. They can be used in parallel;
8. Since the battery does not contain heavy metals like cadmium, lead, or mercury, it is environmentally friendly and considered the most advanced green battery worldwide;
9. They are costly. Lithium batteries are more expensive than other rechargeable batteries.
Third, the internal structure of lithium batteries:
Lithium batteries typically come in two shapes: cylindrical and rectangular.
The battery’s interior adopts a spiral-wound structure, consisting of a positive and negative electrode separated by a very fine, highly permeable polyethylene film as an insulating material. The positive electrode comprises a lithium cobalt oxide collector and an aluminum film current collector. The negative electrode is composed of a sheet-like carbon material collector and a copper film current collector. The battery is filled with an organic electrolyte solution. Additionally, safety valves and PTC components are included to protect the battery from damage during abnormal conditions and output short circuits.
The voltage of a single lithium battery cell is 3.6V, and the capacity is not infinite. Therefore, single lithium battery cells are often connected in series or parallel to meet various requirements. For instance, connecting five cells in series provides a higher voltage output.
Fourth, the charging and discharging requirements of lithium batteries:
1. Lithium battery charging: Considering the structural characteristics of lithium batteries, the maximum charging termination voltage should be 4.2V, and overcharging must be avoided. Otherwise, excessive lithium ions could be extracted from the positive electrode, leading to battery failure. The charging and discharging requirements are stringent, and a dedicated constant current and constant voltage charger should be used. Typically, the battery is first charged at a constant current until it reaches 4.2V per cell, then switched to constant voltage charging. When the constant voltage charging current drops below 100mA, charging should cease.
The charging current (mA) should be between 0.1 and 1.5 times the battery capacity (for example, a 1350mAh battery can have a charging current controlled between 135 and 2025mA). The normal charging current can be approximately 0.5 times the battery capacity, resulting in a charging time of about 2 to 3 hours.
2. Lithium battery discharging: Due to the internal structure of lithium batteries, not all lithium ions can move to the positive electrode during discharge, and some must remain at the negative electrode to ensure smooth insertion during subsequent charging. Otherwise, the battery life will be shortened. To maintain some lithium ions in the graphite layer after discharge, the minimum discharge termination voltage must be strictly limited, meaning lithium batteries should not be over-discharged. The typical discharge termination voltage is 3.0V per cell, with a minimum of 2.5V per cell. The length of battery discharge depends on the battery capacity and discharge current. The formula for calculating discharge time is: battery capacity divided by discharge current. The lithium battery discharge current (mA) should not exceed three times the battery capacity (for example, for a 1000mAh battery, the discharge current should be strictly controlled within 3A), otherwise the battery will be damaged.
Currently, the lithium battery packs available on the market are equipped with matching charging and discharging protection boards. External control of the charge and discharge current is sufficient.
Fifth, the lithium battery protection circuit:
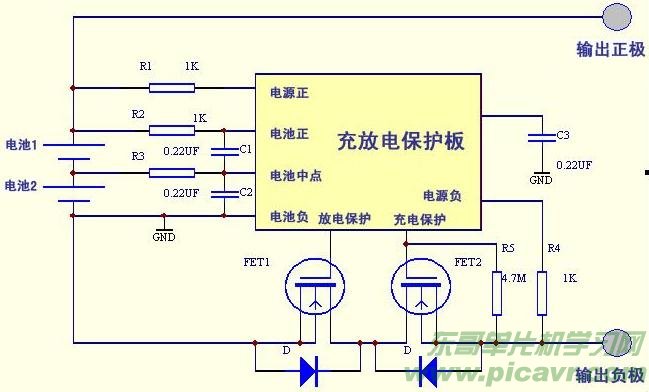
The charge and discharge protection circuit for two lithium batteries is illustrated in Figure 1. It comprises two FETs and a dedicated protection integrated block S–8232. The overcharge control transistor FET2 and the over-discharge control transistor FET1 are connected in series in the circuit. The protection IC monitors the battery voltage and switches off the overcharge protection transistor FET1 when the battery voltage rises to 4.2V to stop charging. To prevent malfunctions, a delay capacitor is typically added to the external circuit. When the battery is discharged and the voltage drops to 2.55V, the over-discharge control transistor FET1 is turned off, halting power supply to the load. Overcurrent protection involves turning off FET1 to stop discharging to the load when a large current flows through the load, protecting both the battery and the FET. Overcurrent detection uses the on-resistance of the FET as a sense resistor, monitoring its voltage drop, and stopping discharge when the voltage drop exceeds the set value. A delay circuit is also generally added to distinguish between inrush current and short-circuit current. While the circuit is fully functional and reliable, it is professional and the dedicated integrated block is not easily purchasable, making it challenging for amateurs to replicate.
Sixth, a simple charging circuit:
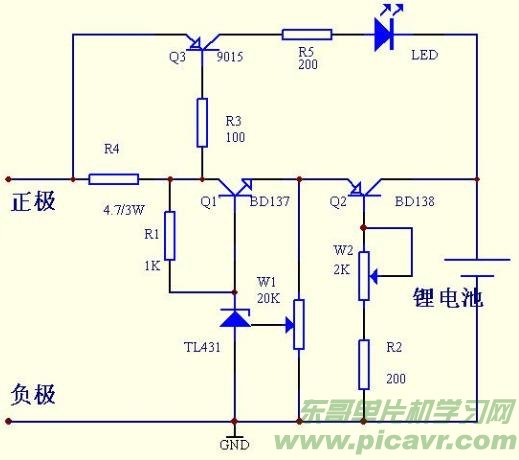
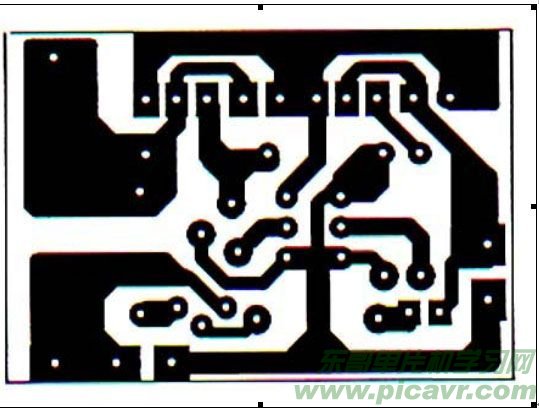
Many merchants now sell single-cell lithium batteries without a charging pad. Their excellent performance and affordable prices make them ideal for replacing homemade products and lithium battery packs, greatly appealing to electronics enthusiasts. Interested readers can refer to Figure 2 to build a charging board. The principle involves charging the battery with a constant voltage to avoid overcharging. The input DC voltage should be higher than the charged battery voltage by 3 volts. R1, Q1, W1, and TL431 form a precision adjustable voltage regulator circuit, Q2, W2, and R2 form an adjustable constant current circuit, and Q3, R3, R4, R5, and LED constitute the charging indication circuit. As the rechargeable battery voltage increases, the charging current gradually decreases. Once the battery is fully charged, the voltage drop across R4 decreases, causing Q3 to turn off and the LED to extinguish. To ensure the battery is fully charged, continue charging for another 1-2 hours after the indicator turns off. Use an appropriate heatsink for Q2 and Q3 when using the circuit. The advantages of this circuit include simple construction, easy component procurement, safe charging, intuitive display, and no risk of damaging the battery. Adjusting W1 allows charging of multiple series lithium batteries, and altering W2 enables adjusting the charging current over a wide range. The downside is the absence of an over-discharge control circuit. Figure 3 is the printed circuit diagram of the charging board (a perspective view from the component side).
Seven, examples of single-cell lithium battery applications:
1. For battery repair and replacement:
There are many battery packs, such as those used in laptops. After repairs, these battery packs are often damaged due to issues with individual cells. In such cases, a suitable single-cell lithium battery can be used as a replacement.
2. Making a highlight flashlight:
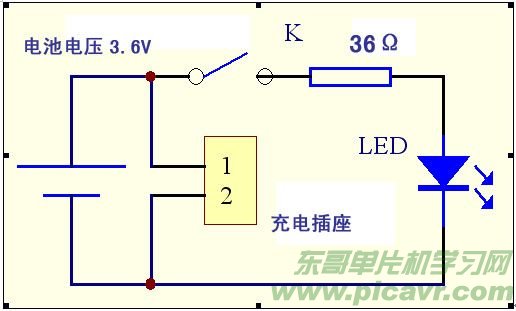
I used a single 3.6V, 1.6Ah lithium battery with a white ultra-high brightness LED to create a mini flashlight. It is easy to use, compact, and aesthetically pleasing. Moreover, thanks to the large battery capacity, with an average nightly use of half an hour, the flashlight hasn't needed recharging for over two months. The circuit is shown in Figure 4.
3. Replacing a 3V power supply:
Since the voltage of a single lithium battery is 3.6V, it can replace two regular batteries, providing power for small household appliances like radios, Walkmans, and cameras. This results in a lighter weight and longer continuous usage.
Eight, the storage of lithium batteries:
Lithium batteries should be fully charged before storage and can be stored for more than six months at 20°C. This indicates that lithium batteries are well-suited for storage at low temperatures. It has been suggested that placing rechargeable batteries in the freezer of a refrigerator is a good idea.
Nine, precautions for use:
Lithium batteries must not be dissected, drilled, punctured, sawed, pressured, or heated, as severe consequences may result. Lithium batteries without a charging protection plate should not be short-circuited and are not suitable for children to play with. Keep them away from flammable materials or chemicals. Proper disposal of disposable lithium batteries is essential.
Fourth, the charge and discharge requirements of lithium batteries:
1. Lithium battery charging: Based on the structural characteristics of lithium batteries, the maximum charging termination voltage should be 4.2V, and overcharging must be avoided. Otherwise, too many lithium ions might be extracted from the positive electrode, rendering the battery unusable. The charging and discharging requirements are strict, and a dedicated constant current and constant voltage charger should be used. Typically, the battery is first charged at a constant current until it reaches 4.2V per cell, then switched to constant voltage charging. When the constant voltage charging current drops below 100mA, charging should stop.
Charging current (mA) should be between 0.1 and 1.5 times the battery capacity (for example, a 1350mAh battery can have a charging current controlled between 135 and 2025mA). The normal charging current can be approximately 0.5 times the battery capacity, resulting in a charging time of about 2 to 3 hours.
2. Lithium battery discharging: Due to the internal structure of lithium batteries, not all lithium ions can move to the positive electrode during discharge, and some must remain at the negative electrode to ensure smooth insertion during subsequent charging. Otherwise, the battery life will be shortened. To keep some lithium ions in the graphite layer after discharge, the minimum discharge termination voltage must be strictly limited, meaning lithium batteries should not be over-discharged. The typical discharge termination voltage is 3.0V per cell, with a minimum of 2.5V per cell. The length of battery discharge depends on the battery capacity and discharge current. The formula for calculating discharge time is: battery capacity divided by discharge current. The lithium battery discharge current (mA) should not exceed three times the battery capacity (for example, for a 1000mAh battery, the discharge current should be strictly controlled within 3A), otherwise the battery will be damaged.
Currently, the lithium battery packs available on the market are equipped with matching charging and discharging protection boards. External control of the charge and discharge current is sufficient.
4Lan Software Router,4Lan Mini Pc,4Lan Firewall Router,4 Ethernet Mini Pc
Shenzhen Innovative Cloud Computer Co., Ltd. , https://www.xcypc.com